Definition
Compounds having same molecular formula but differ in atleast one physical or chemical or biological properties are called isomers and this phenomenon is known as isomerism.
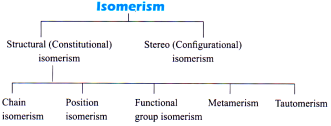
Structural Isomerism
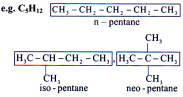
Chain Isomerism
Same molecular formula but differ in the order in which carbon atoms are bonded to each other.
Condition:
I. Size of main chain or side chain or both should be different.
II. Nature of functional group, multiple bond or substituent should not change.
Functional Group Isomerism
Same molecular formula but different functional groups in their molecules show functional group isomerism.
Condition:
Functional group should be different, chain and position isomerism are not considered.

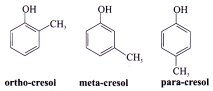
Position Isomerism
Same molecular formula but they differ in the position of the functional group or the substituent.
Condition:
I. Same size of main chain or side chain but different position of group or substituents or functional group.
Metamerism
Metamerism is due to the difference in the nature of alkyl groups attached to the polyvalent atom or functional group. Metamers belong to the same homologous series.
Condition:
I. Functional group should be polyvalent, heteroatomic.
II. Nature of functional group should not change.
III. Chain and position isomerism is not considered
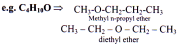
Tautomerism
Tautomerism is the phenomenon in which two structural isomers differ in the relative positions of their atoms and are spontaneously interconvertible and can exist in dynamic equilibrium. In tautomerism two compounds have different functional groups due to shifting of H-atom.
Structural Requirement for tautomerism
I. Compound should have electron withdrawing groups such as -CO, -NO2 and -CN
II. Compound should have atleast one acidic hydrogen on a-carbon of the molecule.
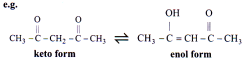
Enol Content Enhanced by:
Acidic a-H of keto form
Aromatisation in enol form
Resonance in enol form
Intra-molecular H-bonding in enol form.
Ring Chain Isomerism
One isomer is open chain but another is cyclic
Ex: Propene and cyclopropane
Stereo Isomerism
Compounds with the same molecular formula and structural formula but having difference in the spatial arrangement of atoms of groups in 3D space are called stereoisomers and the phenomenon is called stereoisomerism. (Spatial – arrangement in space)
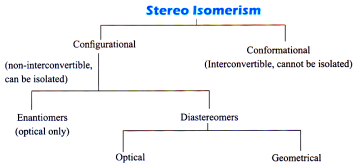
Configuration Isomerism
i. These isomers differ in the configuration (The spatial arrangement of atoms that characterises a particular stereoisomer is called its configuration).
ii. Configurational isomerism arises due to non-interconvertibility at room temperature. Since they are non-interconvertible they can be separated by physical or chemical methods.
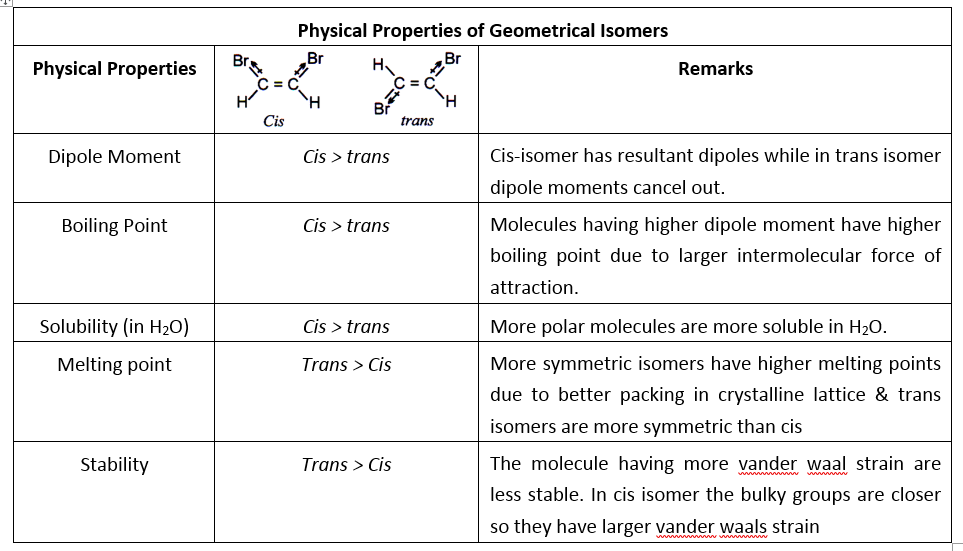
Geometrical Isomerism
Isomers which possess the same molecular and structural formula but differ in the arrangement of atoms or groups in space due to restricted rotation are known as geometrical isomers and the phenomenon is known as geometrical isomerism.
Conditions of geometrical isomerism:
Geometrical isomerism arises due to the presence of a double bond or a ring structure.
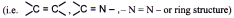
Due to the rigidity of double bond or the ring structure to rotate at the room temperature the molecules exist in two or more orientations. This rigidity to rotation is described as restricted rotation / hindered rotation / no rotation.

Different groups should be attached at each double bonded atom. For example are identical but not geometrical isomers.
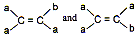
On the other hand, following types of compounds can exist as geometrical isomers:
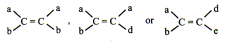
Sequence rules: (Cahn – Ingold – Prelog Sequence Rules) (CIP)
For deciding the seniority of groups following rules are applied:
Rule I : The group with the first atom having higher atomic number is senior. According the this rule the seniority of atoms is I > Br > Cl > S > F > O > O > C > H
Rule II : The higher mass isotope is senior.
Thus (a) -T > -D > -D (b) -C14H3 > – C12H3
Rule III : If the first atom of group is identical then second atom is observed for seniority.
Rule IV : Groups containing double or triple bonds are assigned seniority as if both atoms were duplicated or triplicated.
Rule V : Bond pair is senior to lone pair.
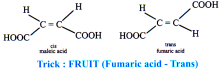
Cis-trans isomerism: The cis compound is the one with the same groups on the same side of the bond, and the trans has the same groups on the opposite sides. Both isomers have different physical and chemical properties.

Optical Isomerism
Compounds having similar molecular and structural formula but differing in the stereo chemical formula and behavior towards plane polarized light are called optical isomers. Isomers and this phenomenon is called optical isomerism.
Types of optical isomers
(1) Optically active (2) Optically inactive
dextrorotatory (d) meso
laevorotatory (l)
Condition:
Molecule should be asymmetric or chiral i.e., symmetry elements (POS & COS) should be absent.: [ POS-Plane of symmetry, COS – Centre of Symmetry]
The carbon atom linked to four different groups is called chiral carbon.
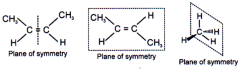
Plane of symmetry
It is an imaginary plane which bisects the molecule in two equal halves in such a way that each half of the molecule is the mirror image of the other half.
Centre of symmetry
The centre of symmetry is defined as the point in a molecule through which if a straight line is drawn from any part of the molecule this line encounters identical groups at equal distances in opposite direction.

Projection formula in optical isomerism
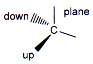
(i) Wedge-dash projection formula
It is a convenient way of depicting three dimensional structure in two dimension. In this projection four bonds of a tetrahedral molecule is shown by two lines (in the plane) one wedge (up the plane) one dash (down the plane)
(II) Fischer projection formula
It is also a convenient way of depicting three dimensional structure in two dimension.
Rules for writing Fischer projection formula:
(i) The molecule is drawn in the form of cross (+) with the chiral carbon at the intersection of horizontal & vertical lines.
(ii) On vertical line, main chain is taken with first carbon at the top.
(iii) The horizontal lines represent the bonds directed towards the viewer and vertical lines away from the viewer
Configurationally nomenclature in optical isomers
Relative configuration
The experimentally determined relationship between the configurations of two molecules, even though we may not know the absolute configuration of either. Relative configuration is expressed by D-L system.
Absolute configuration
The detailed stereochemical picture of a molecule, including how the atoms are arranged in space. Alternatively the (R) or (S) configuration at each chirality centre.
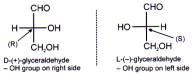
D – L System
This method is used to relate the configuration of sugars and amino acids by the help of enantiomers of glyceraldehyde. The configuration of (+) -glyceraldehyde has been assigned as D and the compounds with the same relative configuration are also assigned as D, & those with (-) glyceraldehyde are assigned as L.
R and S configurations in Fischer projection
Rule I: The priorities of groups which are attached with the asymmetric C-atom are assigned by sequence rule.
Rule II: The lowest priority group is brought to the bottom of Fischer projection by two or even simultaneous exchanges.
Rule III: Then an arrow is drawn from first priority group to second priority group to third priority group. If the arrow is clockwise the configuration assigned to the projection is R & If it is anticlockwise the configuration assigned is S.
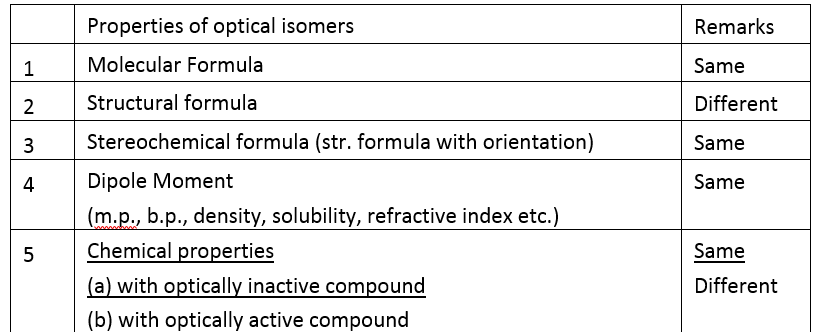
Non-superimposable mirror images are called enantiomers which rotate the plane polarized light up to same extent but in opposite direction.
Diastereomers are stereoisomers which are not mirror images of each other. They have different physical and chemical properties.
Meso compounds are those compounds whose molecules are superimposable on their mirror images inspite of the presence of asymmetric carbon atom. (internal compensation)
An equimolar mixture of the enantiomers (d & I ) is called racemic mixture. The process of converting d or I form of an optically active compound into racemic form is called racemisation. (External compensation)
The process by which d,l mixture is separated into d and I form with the help of chiral reagents or chiral catalyst is known as resolution.
Compound containing chiral carbon mayor may not be optically active but show optical isomerism
For optical isomerism only chiral carbon is not the necessary condition.
Calculation of number of optical isomers

* Where n = no. of chiral carbon
Conformational isomerism
The different arrangement of atoms in space that results from the carbon-carbon single bond free rotation by 0-360° are called conformations or conformational isomers or rotational isomers and this phenomenon is called conformational isomerism.
Conformations
Different arrangements of atoms that can be converted into one another by rotation about single bonds are called conformations.
Conformational isomers
There are infinite arrangement (conformations) which arise due free rotation around carbon-carbon (J bond, different conformations corresponding to energy minima are called conformational isomers. The conformational isomerism arises due to free rotation along a bond.
Newman projection
For conformational analysis, a special type of structural formula is convenient to use which is called Newman projection formula and another type is a Sawhorse formula.
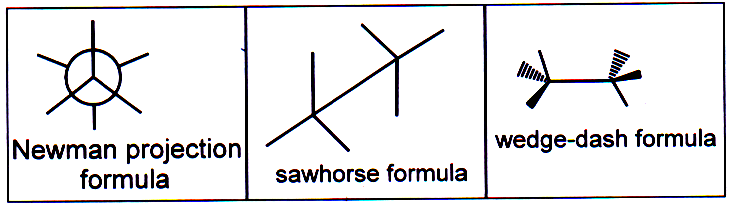
To write Newman projection formula we imagine ourselves taking a view from one carbon atom directly along the selected bond axis to the next atom. The front carbon and its other bonds are represented as and those of the back carbon
Dihedral angle:
The angle between C – X and C – Y in X – C – C – Y when it is visualised along C – C bond.
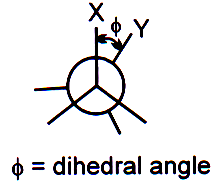
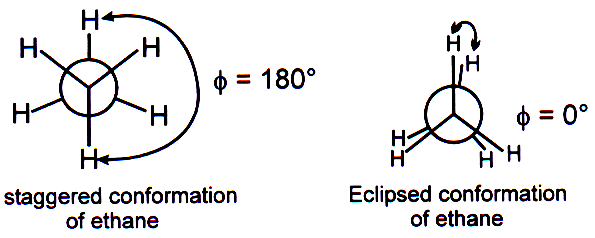
Staggered, eclipsed and skew conformations:
(I) The staggered conformation of a molecule is that conformation where the dihedral angle between the bonds at each atom of carbon-carbon bond is 1800.
(II) In eclipsed conformation the atoms bonded to carbons at each end of carbon-carbon bond are directly opposite to one another. The dihedral angle between them is 00.
(III) Skew conformation : All conformations other than staggered or eclipsed are skew conformations.
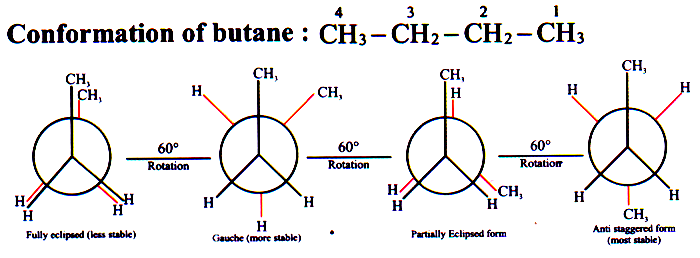
The order of stability of conformations of n-butane
Anti staggered > Gauche > Partially eclipsed > Fully eclipsed.
Relative stability of various conformation of cyclohexane is
Chair > twisted boat > boat > half chair
6 thoughts on “ISOMERISM”