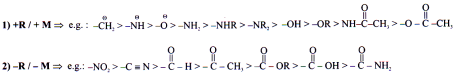
Electronic Effects
The effect which appears due to electronic distribution is called electronic effect.
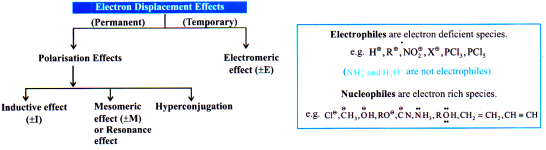
Inductive Effect
Inductive effect may be defined as a permanent shifting of s bond pair electrons due to difference in Electronegativity. (Polar bond).
– I Effect
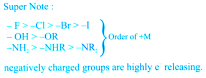
The group which withdraws electron cloud is known as -I group and its effect is called -I effect. Various groups are listed in their decreasing -I strength as follows.
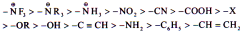
+I Effect
The group which release electron cloud is known as +I group and effect is +I effect.
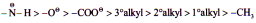
Key Note :-
The hydrogen atom is reference for +I and -I series. The inductive effect of hydrogen is assumed to be zero.

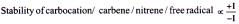


Resonance & Mesomeric effect
Resonance is the phenomenon is which two or more structures involving identical position of atom, can be written for a particular species, all those possible structures are known as resonating structures or canonical structure.
The ultimate target of resonance is to find the real structure of molecule or ion.
Resonance Hybrid
It is the actual structure of the species without violating the rules of covalence maxima for the atoms.
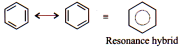
Resonance Energy
The difference in energy between the hybrid and the most stable canonical structure is referred to as the resonance energy.
Relative stability of the canonical form
Nonpolar (uncharged) structure are most stable. Charge separation decreases stability. Separating opposite charges requires energy. Therefore, structures in which opposite charges are separated have greater energy (lower stability) than those that have no charge separation.
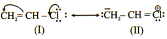
Structures in which all of the atoms have a complete valence shell of electrons (i.e., the noble gas electronic configuration) are especially stable and make large contributions to the hybrid.
Structures with more covalent bonds are more stable than other structures.
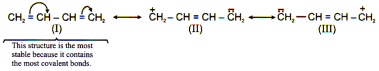
Structure that carries negative charge on a more electronegative atom and positive charge on less electronegative atom are comparatively more stable.
Key Notes :-
The structure with incomplete octet is least stable among all, and charge separation decreases stability.
Charge separation is endothermic process, which increases energy and decreases stability of R.S.
-M Effect
When the group withdraws electron from the conjugated system, it shows -m effect.
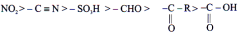
+M Effect
When the group donates electron to the conjugated system it shows +m effect.
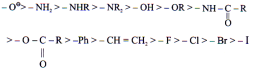
Hyperconjugation
It is delocalisation of sigma electron with p-orbital. Also known as s-p conjugation or no bond resonance. It may take place in alkene, alkynes, carbocation, free radical, benzene nucleus.
Key Note :-
Presence of at least one a-hydrogen in Alkenes, Alkynes, carbocations, free-radical and benzene nucleus is necessary for operation of hyperconjugation.

Applications of hyperconjugation
(a) Stability of Alkenes: “More alkylated alkenes are more stable”. Stability of alkenes µ no. of hyperconjugative structures.
(b) Heat of hydrogenation: Stability of alkenes µ no. of hyperconjugative structure
(c) Bond Length (d) Dipole moment (e) Stability of carbocation (f) Stability of free radical
Relative Stability Order
(A) Stability of carbocation
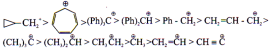
(B) Stability of free radical
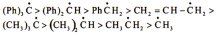
(C) Stability of Carbanion
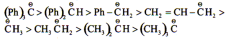
Some important orders in organic chemistry
Basic strength of amine:
In aqueous medium
R Þ -CH3 20 > 10 > 30 > NH3
R Þ -CH2CH3 20 > 30 > 10 > NH3
In gaseous medium:
R Þ -CH3 30 > 20 > 10 > NH3
R Þ -CH2CH3 30 > 20 > 10 > NH3
Reactivity towards nucleophile (NAR)
(1) HCHO > CH3CHO > (CH3)2CO
(2) CCl3CHO > CHCl2CHO > CH2ClCHO
Reactivity order towards acyl nucleophilic substitution reaction
Acid chloride > anhydride > ester > amide
Order of electronic effect
Mesomeric > Hyperconjugation > Inductive effect
Stability of alkene µ no. of a hydrogen
R2C = CH2 >R2C = CHR > R2C = CH2 >
RCH = CHR > RCH = CHR
Trans form cis form
RCH = CH2 > CH2 = CH2
Heat of hydrogenation *
Some important order of acidic strength
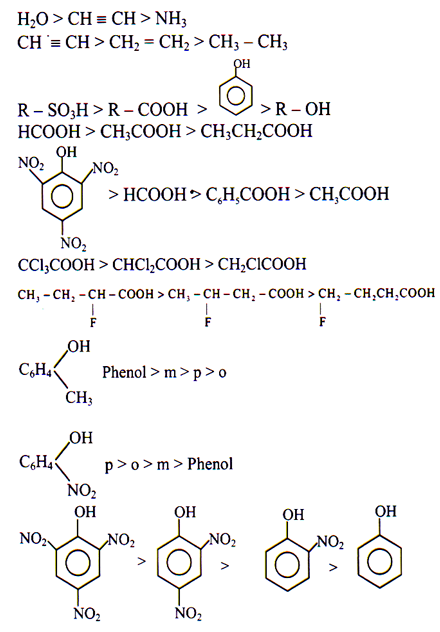
Ortho Effect
Acidic Strength
It is common observation that generally ortho substituted benzoic acids are highly acidic as compared to their isomers and benzoic acids itself.
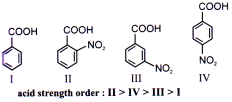
Basic Strength:-
Ortho-substituted anilines are mostly weaker bases than aniline itself.
Ortho-substituent causes steric hinderance to solvation in the product (conjugate acid i.e. cation).
Carbenes
There is a group of intermediates in which carbon forms only two bonds. These neutral divalent carbon compounds are called carbenes.
Nitrenes
There is a group of intermediates in which nitrogen forms only one bond. These neutral Monovalent nitrogen compounds are called nitrenes.
Benzyne
The benzene ring has one extra C – C m bond in benzyne
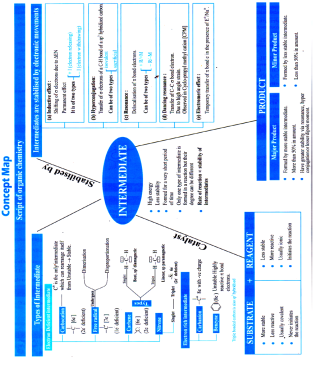
Methods to stabilize Intermediate
Intermediates are stabilised by movement of electrons
Electronic movement is of two types
Permanent electron movement
Inductive Effect
Shifting of s electrons due to DEN
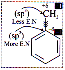
It is of two types
(1) +I Effect Þ
Eg. –NH- > -O- > -COO- > –30R >
– 20R > -10R > -CH3
(2) –I Effect Þ
Eg. NF3+ > NR3+ > NH3+ > NO2 > CH >
F > Cl > Br > I > OH > OR > H
Inductive effect is defined with respect to hydrogen.
Inductive effect of protium is zero while deuterium has +I effect.
Negatively charged groups have very high tendency to release electrons.
Hyper-Conjugation
Transfer of s electrons of a C-H bond of a sp3 hybridized carbon.
Also called “no bond resonance”, Baker Nathan effect.
Can be of two types
(1) isovalent (2) sacrificial
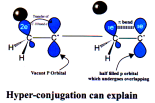
Stability of alkenes
Stability of carbocation & free radical
Activation of benzene ring by alkyl group, methyl is most activating while tertiary being least activating.
[Sacrificial Hyperconjugation]
Resonance
Delocalisation of p bond electrons
Also known as mesomeric effect.
Presence of conjugate system is must
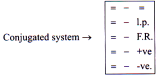
It is of two types:
Effectiveness of various effects: Dancing resonance > Resonance > Hyper conjugation > Inductive effect
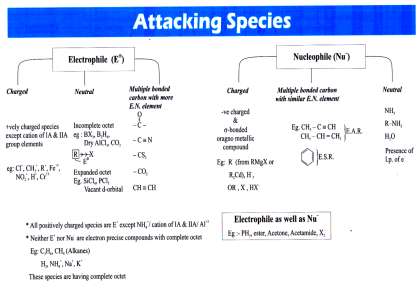
Organic Reaction
Addition Reaction
EAR-Electrophilic Addition Reaction
* bond electrons are mobile in nature and donated to electrophile.
In first step addition of electrophile takes place, that’s why known as EAR.
Compound with multiple bond between similar atoms.
E.g.: Alkene, Alkyne
NAR-Nucleophilic Addition Reaction
Multiple bonds are present in b/w different atoms having difference in E.N.
In first step addition of nucleophile takes place.
E.g.: Addition Reaction of aldehyde, ketone and Cyanides.
FRAR-Free Radical Add. Reaction
Metal adsorb hydrogen on its surface and converts molecular H2 into atomic H. (H2 ® H· + H·)
Eg. 1: Reduction by H2 in the presence of metal (Ni, Pd, Pt)
Eg.2 : Alkene + HBr in presence of peroxide.
Follow anti-markonikov’s rule (HBr + Peroxide)
Substitution Reaction
ESR-Electrophilic Substitution Reaction
Characteristic Reaction of benzene and its derivatives to maintain aromaticity.
Substituted E+ is always H+.
Protonic acids like HCl, HF etc does not directly react with benzene.
Eg.: Friedel craft Alkylation and Friedel craft Acylation (Anhy. AlCl3 Produces electrophile), and other reaction of benzne and its derivatives.
FRSR-Free Radical Substitution Reaction
Free radical substituted by other free radical.
Generally in alkanes.
30H > 20H > 10H (Reactivity order)
Eg.: Halogenation of alkanes (X· replace H·)
NSR-Nucleophilic Substitution Reaction
Nucleophile is substituted by another nucleophile.
SN1 Þ 30R-X > 20R-X >10R-X
SN2 Þ 10R-X > 20R-X > 30R-X
Ar SN Þ Ph-X
SNAE Þ Acid derivatives.
Elimination Reaction
E1-Elimination Reaction
I.M. is Carbocation and can undergo rearrangement.
1st Order Reaction
R.O.R. µ [Substrate]
30 > 20 > 10.
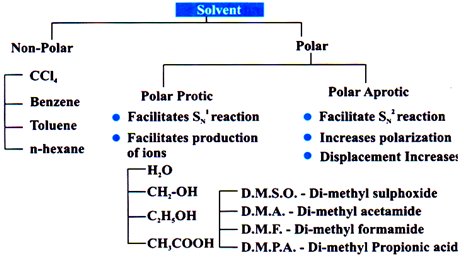
Polar protic solvent and high temperature is required.
Eg.: Dehydration of alcohols.
E2-Elimination Reaction
No carbocation is formed.
2nd order reaction
R.O.R. µ [Substrate] [Nu–]
10 > 20 > 30.
In presence of strong Nu–
Substrate should be sterically crowded.
Eg.: Dehydrohalogenation.
Elimination unimolecular conjugate base reaction E1-CB
R.O.R. = [C.B]1
Most stable carbocation is formed but not rearrangement occurs.
Oxidation Reaction
Oxidation Number Increases
Via Abstraction of Hydrogen
By weak/moderate oxidizing agent
PCC, PDC, CrO2Cl2, Cu/573 K Jones reagent – [CrO3 + dil. H2SO4 + acetone]
Via Introduction of Nascent [O]
Strong oxidizing agent.
Eg.: KMnO4, K2Cr2O7, CrO3, O3.
Reduction Reaction
Oxidation Number Decreases
Via adding of Hydrogen [H+]
(Pd + CaCO3)/H2
Ni/Pt/Pd + H2
Via introducing of Hydride [H–]
Li A lH4 (stronger reducing agent) NaBH4.
Di-BAL-H
(diisobutylaluminium hydride)
[SnCl2 + HCl] (H– + Sn+4 + 3Cl–)
Acid Base Reaction
*Most Important Reaction
*90% students get confused by this reaction
70% reactions of organic chemistry are acid base reactions.
Fastest Reactions.
Salt is produced in this reaction and anionic part of salt can act as Nucleophile.
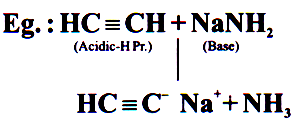
Other Reaction
Peri-Cyclic Reaction
Polymerisation Reaction
Rearrangement Reaction
Aromatization Reaction
Condensation Reaction
Isomerisation Reaction
Combustion Reaction
Simplified Addition Reaction

Simplified Substitution Reaction

Selectivity in the Reaction
Chemo-Selective
When reaction occurs at a specific functional group.
Selectivity in the Reaction
Regio-Selective
Regio = Region
There Reaction are Region specific Reactions
Selectivity in the Reaction
Stereo-Selective
One of the stereo isomer is the main product.
Selectivity in the Reaction
Stereo-Specific
Product of this reaction depends on the stereo chemical of substrate.
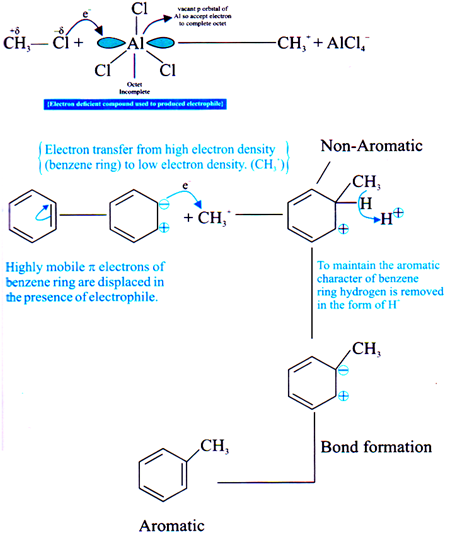
Electrophilic Substitution Reaction
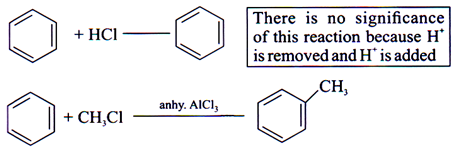
MechanismÞ
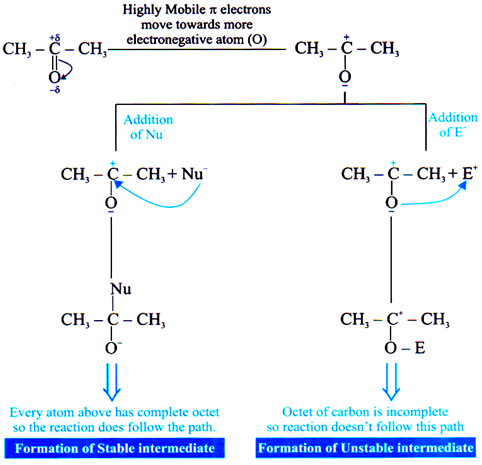
Nucleophilic Addition Reaction
Catalytic Hydrogenation
Homogeneous
Catalyst gets dissolved in reaction mixture.
Eg.: (1) Wilkinson’s catalyst, [Rh(Ph3P)3]Cl
(2) Metal + H2 mixed in quinoline.
Heterogeneous
Eg.: (1) Raney Ni(Ni + Al)
More porous
Size difference in Al and Ni.
Large surface area.
Eg.: (2) Metal + H3
Ni/Pt/Pd/Ni2B

Metal with H+ donor
Birch Reduction (Anti addition)
Liq. NH3 + Na
Bouveault-Blace Reduction
Na + C2H5OH
Stephen’s Reaction
(SnCl2 + HCl)
Clemmensen’s Reaction
[Zn – Hg + HCl]
Step 1– e– released by metal.
Step 2– Abstraction of H+ from H+ donar
Reduction (In Organic Chemistry)
Metal Hydride (H– Releasing)
LiAlH4
It reduces all except Alkene, Alkyne, Benzene
NaBH4
Weak Reducing agent
Specific for carbonyl compound.
DiBAL-H
Specific for ester and cyanide
Used in synthesis of aldehyde
Other
Red P + HI
Oxygen containing functional group (Alkanes [Converts into])
Merrwin Ponndorf Verley reducing
Wolf Kishner’s Reducing
[NH2-NH2, NaOH]
Oxidation of Alkenes/Alkynes
Weak O.A.
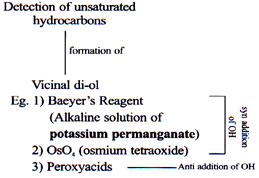
Mild O.A
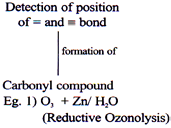
Strong O.A.
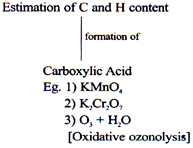
Oxidation of Alcohols
Hydrogen Abstractive O.A. (mild O.A.)/[Limited (O)]
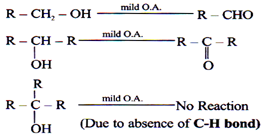
Mild O.A. can reduce only C-H bond containing compounds. C-H bond has tendency to trap [O]
Eg. (1) P.C.C. (Pyridinium chlorochromate): [C5H5NH]+[CrO3Cl]–
(2) PC – Collin’s Reagent
(3) PDC – (Pyridinium di-chromate)
(4) Cu/5000C
(5) CrO3/H2SO4 (Jones’ Reagent)
Oxygen Releasing O.A. (Strong O.A.)
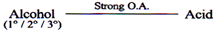
It Oxidises C-H bond as well as C-C bond.
Eg. (1) KMnO4/H+
(2) K2Cr2O7/H+
(3) HIO4
HIO4: (periodic cleavage) It is used for compounds having more than one OH group. Eg.
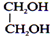
: (periodic cleavage) It is used for compounds having more than one OH group. Eg.
Oxidation of Alkane
Alkanes generally resist oxidation.
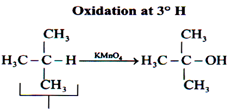
Only alkane which can be oxidized.
Oxidation (In Organic Chemistry)
Oxidation of Carbonyl Compounds
Strong Oxidizing agent
Eg. KMnO4/H+, K2Cr2O7/H+
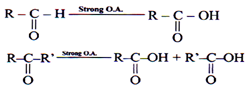
Popoff’s rule- During the oxidation of ketones, keto group always stays with the smaller alkyl group.
Mild Oxidizing agent
Used in differentiation of aldehyde and ketones.
Ketones cannot be oxidized by mild O.A.
Eg. (1) Benedict’s Solution
Sodium Citrate + CuSO4.5H2O
Red ppt. of Cu2O forms
(2) Schiff Reagent
(3) Fehling’s solution
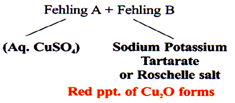
(4) Tollen’s Reagent
It is also known as Silver Mirror Test
Ammonical Silver Nitrate – [AgNO3 + NH4OH]

3 thoughts on “REACTION MECHANISM”